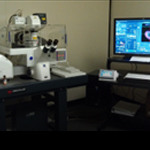
Instrumentation
The Microscopy Core hosts a wide variety of instrumentation for research needs. Browse our systems and instruments.
The Microscopy Core in the Center for Targeted Therapeutics is a core facility that provides access to state-of-the-art biological imaging technologies for University of South Carolina researchers, collaborators and external organizations.
The Microscopy Core, one of three core facilities supported by the COBRE Center for Targeted Therapeutics, offers a variety of imaging technologies, including phase contrast, differential interference contrast (DIC), widefield fluorescence, and confocal microscopy.
We are housed in the Coker Life Sciences Building and serve a large community of researchers studying a wide range of topics in drug discovery and biomedical sciences.

The Microscopy Core hosts a wide variety of instrumentation for research needs. Browse our systems and instruments.
The Microscopy Core offers services to expedite research and experimentation. Learn more about our services offered.
The Microscopy Core has contributed to a variety of projects in drug discovery and biomedical sciences. See samples from our completed projects.