Researchers have identified an important link in the complex question of autism spectrum disorders, thanks to the work of several University of South Carolina neurobiologists.
Sofia Lizarraga, an assistant professor of biological sciences, and her collaborators recently published a paper in Molecular Psychiatry detailing their discovery of the mechanisms underlying a major genetic risk factor for one subtype of ASD, and more significantly, a possible way to reverse it.
The paper, coauthored by Seonhye Cheon and Allison Culver, features research conducted in the Lizarraga laboratory by collaborators at UofSC and Brown University. Their work has recently been spotlighted by Spectrum News, a leading source for news on autism research.
ASD affects one in 44 children, according to the most recent studies. In most cases, the genetic causes of the disorders are unknown; but scientists do know that ASD is associated with differences in neuronal connectivity, which can severely impact brain function, speech and other behaviors.
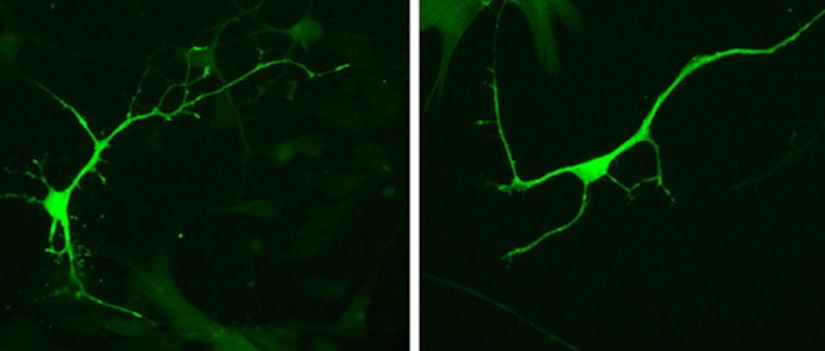
Lizarraga and her research team used human stem cells to look at genetic factors that are associated with the disorders. They used a process called nuclear reprogramming on adult blood or skin cells that allowed them to turn back the clock to generate stem cells.
Using the stem cells that they coached into becoming neurons, Lizarraga examined epigenetic mechanisms they suspected were associated with autism.
Unlike genetic changes, epigenetic changes are reversible and do not change the DNA sequence, but they can change how and when a DNA sequence is expressed or turned on. Mutations in these epigenetic regulators have been associated with autism spectrum disorders.
The families of children with ASH1L mutations are very excited about this research, because they are eager to find a treatment.
— Sofia Lizarraga
Lizarraga and her team discovered that loss of one such regulator, known as ASH1L, regulates gene morphology and maturation — what genes will look like and how neurons will gain their functions.
Individuals with ASH1L mutations present with autistic behaviors, intellectual disabilities, and in some cases, seizures. What Lizarraga found suggests that individuals with ASD with half the normal amount of ASH1L may have impaired neuronal connectivity which could arise from deficits in neuronal morphogenesis or maturation.
Foster Ritchie, a master’s student in biological sciences and contributor on the paper, followed-up on some recent studies identifying ASH1L as potentially having a role in the maturation of the neurons.
"We were able to corroborate that a knock down of ASH1L really disrupts the morphology of the neurons,” Richie says.
The researchers also found evidence that genes affected by the ASH1L mutations could be recovered, meaning they could be restored to typical function.
By targeting a different epigenetic regulator, the Polycomb repressor complex, Lizarraga was able to counteract the effects of the loss of ASH1L by using a Polycomb inhibitor. Similar inhibitors are currently used as FDA-approved treatments for some forms of cancer.
The research is still in its infancy, and Lizarraga says much more work must be done to determine the exact correlation between ASH1L and ASD, as genetic background may play a large role.
“That’s something that we wonder,” Lizarraga says. “Different mutations, even though they’re in the same gene, might affect individuals differently. There is a need to clinically characterize these patients more profoundly.”
For now, Lizarraga’s research has provided a light of hope for families of children severely impacted by ASH1L mutations.
“The families of children with ASH1L mutations are very excited about this research,” Lizarraga says, “because, as you can imagine, they are eager to find a treatment.”